- About US
- Contact Us
- Mediapack
- RSS
Innovation UK > News > Innovation UK Vol5-1 > Innovation - soup to nuts, served by the NIC

Innovation - soup to nuts, served by the NIC
Successful innovation starts with identifying a need. It then grows the solution to that need and shares the results. The NIC can support commercial innovators at each of these stages.
Is there anyone in 2009 who doesn?t have ?innovation? somewhere in their job description? It?s the buzz word of the moment. Ministers have hailed it as the way out of our present economic difficulties, industry tell us it is the route by which we will maintain our competitive edge in the knowledge economy, and every job holder in the land knows that the answer to the problems they face inevitably involve a new way of working or a new bit of kit. Little wonder ?innovate? is the new mantra.
And we?re good at it. In industries as diverse as entertainment, travel and pharmaceuticals, the UK has led the world, even if for just a limited time. Healthcare is no exception. Although our world standing may have ebbed and flowed, we have always been at the cutting edge in terms of creativity ? whether from university medical departments, the healthcare industry or NHS staff themselves.
But many organisations involved in healthcare innovation feel and act like space cadets, floating in the stratosphere and linked up to no-one, least of all the Mother Ship. Because the NHS doesn?t always articulate its priority ?needs?, innovators are compelled to take a leap into the unknown and can?t be sure their solutions address a problem or, at least, not the main problem.
IDENTIFYING THE NEED
But one organisation is tackling these issues. Working in the field of medical devices and diagnostics to deliver innovations, the NHS National Innovation Centre (NIC) starts by looking at the needs of the health service. In partnership with the Technology Strategy Board?s Statement of Clinical Need team, they bring together groups of healthcare professionals to identify the most important problems and deficiencies.
Brian Winn, who heads the NIC, says: ?Innovation has most value when products and services meet a defined clinical need. This proactive approach ? getting out there and talking to the practitioners ? has already yielded huge efficiencies in speeding up the time it takes to get products to the bedside.?
The NIC calls the approach ?Wouldn?t It Be Great If?? (WIBGI), and challenges teams of clinicians, academics and NHS staff, working in specific areas, to identify their priority needs. Last year the NIC proposed to DH that it apply the WIBGI approach to the NHS priority problems of Healthcare Acquired Infections (HCAI)* and the 18-week wait targets in Orthopaedics. Combined with the NIC?s innovative approach to commissioning innovation, the HCAI work resulted in new patient isolation technologies being delivered at least a year earlier than would otherwise have been the case. At the NHS EXPO event on 18 & 19 June, the NIC will be running four new WIBGIs in Urology, Paediatrics, Ambulance Services and Coronary Care*.
But the proactive approach is not the only one. Winn says: ?We do, of course, use ideas that come into us unprompted. But the most successful are always from innovators who have a clear sense of need.?
One example is Cambridge-based company TwisDX, who have developed a method of amplifying DNA so that patients can be tested for MRSA in under 15 minutes before they are admitted to hospital*. The result is that anyone found to be carrying the infection can be isolated immediately, according to the hospital?s infection control procedures. TwisDX received business advice and funding from the NIC to develop their product. At the HCAI conference in Feb 09, the company won the award for ?a new and/or novel HCAI-related product or technology likely or proven to help reduce infection?.
HEALTHCARE ACQUIRED INFECTIONS (HCAI)
In 2008, the NIC used its proactive approach to defining need and developing solutions in the field of Healthcare Acquired Infections (HCAI), a priority workstream being managed for the DH by the NHS Purchasing and Supply Agency.
To define the need, the NIC ran a number of ?Wouldn?t It Be Great If? sessions (WIBGIs) with the Infection Control community across the NHS. The WIBGIs generated over 150 ideas that were then assessed and shortlisted to 50 by the same community. The top ten then received active attention by DH.
To design the solution, the NIC commissioned an external company through a series of staged contracts that awarded finance at pre-determined points in the development. Each contract was carefully managed by the NIC to ensure that each milestone was delivered successfully before going on to the next.
The resulting technologies were demonstrated at the HCAI conference in March 09 to critical acclaim.
DEVELOPING THE SOLUTIONS
Once the clinical needs are agreed, the NIC scans the market to see if a product exists that will meet those needs. If it doesn?t, it embarks on a tightly managed system to develop the solutions. The first step calls for users, designers and manufacturers to produce outline designs for potential devices or diagnostics. In the HCAI work, the NIC-supported the co-development of six products and trialled them in two hospitals.
At the same time, companies approach the NIC with ideas that have potential but might need moderating or strengthening. The NIC offers advice to them via online tools (www.nic.nhs.uk) and offline services. Lein Applied Diagnostics, a Reading-based SME, approached the NIC in 2007 with a prototype for a non-invasive blood glucose meter*. A hand-held device will replace the finger stick methods currently used by people with diabetes to test blood glucose levels and, instead, will take a painless reading from the eye.
Dan Daly, director of Lein, said: ?I put the product through the NIC?s web-based ?Scorecard? to begin with, and carried out a confidential assessment myself of the device using the NIC?s rigorous tools. I then contacted the NIC and asked for further support. Their advice on markets, business strategy, legal requirements and arranging NHS introductions was invaluable.?
TWISDX
The spread of MRSA in hospitals could be curtailed with a quick, on-the-spot test being developed by an emerging company in Cambridge. TwisDX has designed a rapid, portable test for Methicillin-resistant Staphylococcus aureus (MRSA), which is based on a new way of detecting DNA. The technology, called recombinase polymerase amplification or RPA, employs enzymes to amplify small amounts of DNA to detectable levels. When a patient enters a hospital, a swab from their nose is taken and inserted into the device, which is small enough to sit on a nurse?s station.
The test is able to detect MRSA infection within 10-15 minutes. Current methods for detecting MRSA require highly trained personnel and a centralised testing facility, taking many more hours for results.
NON-INVASIVE BLOOD GLUCOSE METER
Reading company, Lein Applied Diagnostics, is developing a hand-held, non-invasive blood glucose meter device that people with diabetes will simply look into it to get a measure of their glucose level. It will eliminate the need for the more painful and cumbersome traditional ?finger stick? solutions. The glucose meter has already successfully achieved technical and clinical targets with independently reviewed results. Clinical testing has shown good correlations with finger stick blood glucose meters and a detailed clinical trial is now underway. It is intended that the final product will be easy to use, non-invasive, painless, have no side effects and will cost less than the current finger stick solution.
DIFFUSING ACROSS THE NHS
Companies and innovators approach the NIC with products at every stage of development, and the NIC project manages that development up until the device is in use. Southampton University Science Park company, VNUS Medical Technologies UK Ltd, had developed a minimally invasive alternative to vein-stripping surgery (www.vnus.com/navigation/vsl.htm) for people who suffer from varicose veins*. The VNUS Closure® procedure is performed under local anaesthetic, with little or no pain, so that patients can walk away and be back to everyday activities within a day. Although the procedure was more cost effective than traditional methods, and the patient benefits were obvious, the company was struggling to break into the NHS. After registering on the innovator website tools, the NIC helped broker introductions for VNUS Medical Technologies for both clinical training and trials. The product and surgical process are now in use in over 40 Trusts.
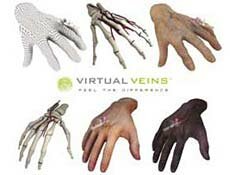
Another example was UK Haptics, a Northumberlandbased technology company that had developed a webbased application, called Virtual Veins, that provides training in venepuncture. Trainees practise on a virtual 3D hand until confident. The lifelike simulation gives the trainee tactile and informative feedback on their performance*. The NIC funded improvements to the simulation model, with the result that it was adopted by NHS training centres.
VNUS CLOSURE®
Southampton University Science Park company, VNUS Medical Technologies UK Ltd, had developed a minimally invasive alternative to vein-stripping surgery for people who suffer from varicose veins. The VNUS ClosureFAST ? Single Patient Use Radiofrequency Catheter, with unique Segmental Ablation Technology?, delivers controlled radiofrequency energy to the vein wall. Unlike other endovenous ablative methods, that depend on a manual pull-back method, this catheter delivers radio frequency energy in seven centimetre segments whilst the catheter is stationary. Segmental Ablation Technology? eliminates problems of uncontrolled and inadequate energy delivery with too-slow or too-fast pull-back. The VNUS ClosureFAST? Catheter can run over a guidewire and is controlled by the surgeon with a built-in energy delivery control switch.
In 2008, the NIC?s innovation management systems and web tools (accredited to ISO9001:2008) attracted the attention of other government departments who had also been charged with developing approaches to harnessing and developing innovation. Under the auspices of DIUS, who lead on innovation for Government, the tools will now be made available to other Government departments via the Government Gateway. The NIC is charged with developing innovation management systems in healthcare. In line with that role, they are currently developing further online refinements and off-line services.
 These will support not only innovators from industry but also the NHS Strategic Health Authorities who are in the front line to fulfil the NHS?s determination to use the best technology for the benefit of patients. Brian Winn says: ?Until they see actual examples put into practice, most people are uncomfortable with the idea that innovation can be proactively stimulated and managed. The NIC and its partners are building the evidence base that this is in fact the case, and making freely available a suite of innovation management tools and processes for others to use across the entire ?Identify, Grow and Diffuse? spectrum. Yes, there is still room for the famous ?Eureka!? moment, but don?t overlook the fact that, when that original Eureka happened, Aristotle was seeking to fulfil a need that had been placed on his shoulders!?
These will support not only innovators from industry but also the NHS Strategic Health Authorities who are in the front line to fulfil the NHS?s determination to use the best technology for the benefit of patients. Brian Winn says: ?Until they see actual examples put into practice, most people are uncomfortable with the idea that innovation can be proactively stimulated and managed. The NIC and its partners are building the evidence base that this is in fact the case, and making freely available a suite of innovation management tools and processes for others to use across the entire ?Identify, Grow and Diffuse? spectrum. Yes, there is still room for the famous ?Eureka!? moment, but don?t overlook the fact that, when that original Eureka happened, Aristotle was seeking to fulfil a need that had been placed on his shoulders!?
* All the innovators and innovations mentioned in this article can be seen at the NHS EXPO event at the ExCel Conference Centre, London, on 18 & 19 June.
Added the 25 August 2009 in category Innovation UK Vol5-1
Related elements :
- Healthcare
-
NHS National Innovation Centre
Life science and healthcare technology industries are to make a major contribution to the future wealth of the UK

social bookmarking








Tags: Key Technology, healthcare, NHS, pharmaceuticals